Ch2o lewis structure
In order to find the total valence electrons in CH2O moleculefirst of all you should know the valence electrons present in carbon atomhydrogen atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom, ch2o lewis structure. Carbon is ch2o lewis structure 14 element on the periodic table. Hydrogen is group 1 element on the periodic table.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Lewis diagrams. About About this video Transcript.
Ch2o lewis structure
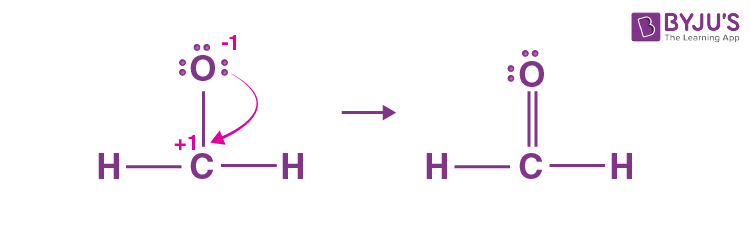
Formaldehyde is an organic compound with the chemical formula CH 2 O that appears as a colourless gas. It is the most common and simplest aldehyde, consisting of two hydrogens, one carbon and one oxygen. Lewis structure diagrams show how many valence electrons are available within an atom and participate in bond formation. It also enables visualising the behaviour of the valence electrons within the molecule and determining whether or not a lone pair of electrons exist. Determine the total number of electrons in the carbon, hydrogen, and oxygen valence shells. Hydrogen is a Group IA element with only one electron in its last shell. Oxygen is a Group VIA element with six electrons in its last shell. Carbon is a Group IVA element with four electrons in its last shell. Determine the total number of electron pairs that exist as lone pairs and bonds. Total electron pairs are calculated by dividing the total valence electron count by two. In the valence shells of the HCHO molecule, there are 6 pairs of electrons. To obtain the best Lewis structure minimise charges on atoms by converting lone pairs to bonds. There are charges on the carbon and oxygen atoms in the centre. We will convert a single covalent bond from a single lone pair of oxygen atoms. The molecular geometry of CH 2 O is trigonal planar because the central carbon atom has no lone pair and is attached to the two hydrogen atoms and one oxygen atom through two single bonds and one double bond.
I recommend you do not attempt to synthesize it, or purchase it.
Formaldehyde, symbolized as CH 2 O, is a simple and widespread organic compound. This colourless gas consists of two hydrogen atoms, one carbon atom, and one oxygen atom. Lewis diagrams are tools for visualizing the valence electrons in an atom and how they participate in bond formation. They also allow us to see if there are any lone pairs of electrons present. These diagrams are formed by drawing electrons as dots, typically in pairs, around the symbol of the atom.
Lewis structures — also called Lewis dot formulas , Lewis dot structures , electron dot structures , or Lewis electron dot structures LEDs — are diagrams that show the bonding between atoms of a molecule , as well as the lone pairs of electrons that may exist in the molecule. The Lewis structure was named after Gilbert N. Lewis , who introduced it in his article The Atom and the Molecule. Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another pairs of dots can be used instead of lines. Excess electrons that form lone pairs are represented as pairs of dots, and are placed next to the atoms. Although main group elements of the second period and beyond usually react by gaining, losing, or sharing electrons until they have achieved a valence shell electron configuration with a full octet of 8 electrons, hydrogen H can only form bonds which share just two electrons. The total number of electrons represented in a Lewis structure is equal to the sum of the numbers of valence electrons on each individual atom. Non-valence electrons are not represented in Lewis structures.
Ch2o lewis structure
The Oxygen atom has 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of CH2O. Here, the given molecule is CH2O. In order to draw the lewis structure of CH2O, first of all you have to find the total number of valence electrons present in the CH2O molecule. Valence electrons are the number of electrons present in the outermost shell of an atom. Carbon is a group 14 element on the periodic table. Hydrogen is a group 1 element on the periodic table. Oxygen is a group 16 element on the periodic table. Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table.
Web drama turkey
The total number of electron pairs is obtained by dividing the total number of valence electrons by two. Structure, Synthesis, and Reactions - Testbook. Carbon is more electropositive than oxygen. We will convert a single covalent bond from a single lone pair of oxygen atoms. Step 1: Determine the total number of valence electrons in the Formaldehyde. Sort by: Top Voted. I understand it is naturally occurring and wondering if there would be a way to safely extract it for use with wet specimens. In the valence shells of the HCHO molecule, there are 6 pairs of electrons. All right, now let's do this together. What Is Charles Law. So let's put the carbon right over here, and then let's put these other atoms around it. But then in the fourth step, we're going to look at our central atom. It has to do with the geometry of the molecule. Hydrogen only forms one single bond in these sort of problems. As shown above, there are already three bonds, and the other three lone pairs should be marked on hydrogen, carbon, and oxygen atoms.
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell.
So let's give it six electrons. The total number of electron pairs is obtained by dividing the total number of valence electrons by two. There is a difference in electronegativity values between carbon and oxygen, which causes charge imbalance and generates some dipole moment in the molecule, making CH 2 O a polar molecule. These hydrogen atoms and oxygen atom are forming a duplet and octet respectively and hence they are stable. It also enables visualising the behaviour of the valence electrons within the molecule and determining whether or not a lone pair of electrons exist. Why is the least electronegative atom the central atom? All right, now let's do this together. Hope that helps. And in this case that would be called a radical. Scroll to Top. And in that shell, it has one, two, three, four valence electrons. Decide on the central atom. The molecular geometry of CH 2 O is trigonal planar because the central carbon atom has no lone pair and is connected to two hydrogen atoms and one oxygen atom through two single bonds and one double bond. Marshall Acid. This indicates that these atoms are chemically bonded with each other in a CH2O molecule.

0 thoughts on “Ch2o lewis structure”