C4h10 lewis structure
Submitted by Eric W. Calculate the pH and pOH of each solution. Show comple stoichiometric solutions and equations.
Lewis structures are drawn using the valence electrons of constituent elements of a compound. Only these electrons participate in bonding and form single, double or triple bonds to satisfy their respective octet except hydrogen. The molecular formula is. The possible compounds with formula are butane and isobutane. Add to cart. Forgot password? Register Now.
C4h10 lewis structure
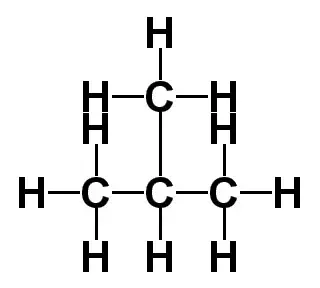
This is the C4H10 Lewis structure: Butane. For Butane, we have a total of 26 valence electrons. Whenever we see the ending, "ane", we know that we're going to have Carbons and Hydrogens single bonded. That makes it a little bit easier to draw the C4H10 Lewis structure. We'll put four Carbons in a row and then we'll put Hydrogens around them. Because each Carbon needs to have four single bonds--each bond having two valence electrons, that'll give it an octet--we'll have three Hydrogens on the end Carbons and two on the center, like this. There are the three on the ends, and then we'll put two Hydrogens on the central Carbons. Next we'll place a single bond between each of the atoms to show that a pair of electrons is being shared. So we've used all 26 valence electrons for the C4H10 Lewis structure, and we can see that each Carbon has four single bonds. Since each single bond has two valence electrons, that means that each Carbon has an octet.
Try Numerade free for 7 days View This Answer. Already have an account?
Submitted by Stefanie M. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. Draw the Lewis dot structure for butane. How many electron domains does the molecule have?
C 4 H 10 butane has four carbon atoms and ten hydrogen atoms. In the C 4 H 10 Lewis structure, there are three single bonds between the four carbon atoms. The left carbon and right carbon are attached with three hydrogen atoms, and the two center carbons are attached with two hydrogen atoms. And none of the atoms has a lone pair. In the periodic table , carbon lies in group 14, and hydrogen lies in group 1. Hence, carbon has four valence electrons and hydrogen has one valence electron. Learn how to find: Carbon valence electrons and Hydrogen valence electrons. We have a total of 26 valence electrons. And when we divide this value by two, we get the value of total electron pairs.
C4h10 lewis structure
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot diagram or electron dot diagram, or a Lewis diagram, or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. The order in which the positions are used does not matter.
12497 coach position
So we're done with the Lewis structure for C4H10, Butane. F09 [DBID]. Draw the structural formula of butane. Indicate hybridizations and bond angles at each carbon atom. Notes Access past notes and exams matches to your classes Study Groups Study with your friends by joining virtual study sessions Free Unlocks Download the mobile app and receive 3 free video solutions. Video Answer Solved by verified expert. Hydrogen is group 1 element on the periodic table. Suggested Textbook. Note that Hydrogen only needs two valence electrons to have a full outer shell. In the above lewis dot structure of C4H10, you can also represent each bonding electron pair : as a single bond. C Stoichiometry is best defined as the quantitative relationship between reactants and products in a chemical reaction. Explain why the carbon atom chain is not straight.
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History.
The pH of a solution is 8. Draw the structural formula of butane. Indicate hybridizations and bond angles at each carbon atom. Notes Access past notes and exams matches to your classes Study Groups Study with your friends by joining virtual study sessions Free Unlocks Download the mobile app and receive 3 free video solutions. Colorless gas with a gasoline-like or natural gas odor. The four Carbon atoms C are at the center and they are surrounded by the Hydrogen atoms H. In the above lewis dot structure of C4H10, you can also represent each bonding electron pair : as a single bond. The answer lacks proper grammar and punctuation. Draw the Lewis dot structure for butane. Notes Access past notes and exams matches to your classes Study Groups Study with your friends by joining virtual study sessions Free Unlocks Download the mobile app and receive 3 free video solutions. The balance initially reads D A chemical is placed on a balance in an airtight container. Where more than one resonance structure is important, give examples of all major contributors. The C4H10 molecule has a total of 26 valence electrons and all these valence electrons are used in the above sketch of C4H The molecule is drawn as a circle, with the carbon atoms at the center and the hydrogen atoms surrounding them.

Thanks for an explanation. I did not know it.
Amusing state of affairs