C2h6 electron dot structure
Write electron dot structure of ethane molecule C 2 H 6. Electron dot structure of ethane molecule C 2 H 6. Write the : a molecular formula, b electron dot formula and c structural formula of methane and ethane. Byju's Answer.
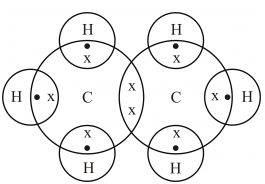
Carbon is the central atom, hydrogen is the outer atom, there is a single bond between the two carbon atoms, each carbon atom is connected to three hydrogen atoms by a single bond, and none of the atoms have a lone pair of electrons. The C2H6 Lewis structure is shown below:. Carbon and hydrogen are group 14 and group 1 elements in the periodic table. The central atom must satisfy the principle of less electronegativity. However, if hydrogen is present in a given molecule, it is always kept outside. So for the C2H6 or ethane molecule, even though the hydrogen atoms are less electronegative than the carbon atoms, we must leave the hydrogen on the outside. Thus, the carbon atom C is the central atom and the hydrogen atom H is the outer atom.
C2h6 electron dot structure
Since C has 4 valence electrons , and each H atoms contributes 1 valence electron, the total number of electrons will be. So, the two C atoms are placed in the center of the molecule. Each C atom forms three covalent bonds with three H atoms, with one aditional covalent bond being formed between the two C atoms. Each of these seven single bonds contains 2 electrons, which means that a total of. How can I write the Lewis dot structure for C2H6? Stefan V. Jan 11, Related questions What are lewis dot structures used for? How do you draw the lewis structure for ions? How do you draw the Lewis structure for ionic compounds? What are some examples of Lewis structures? What is an example of a Lewis structures practice problem?
Step 4 Stability of structure In Step 3 we can see that the external hydrogen atoms in the C2H6 molecule are forming a bimolecule, so they are stable. How can I draw a Lewis structure of a compound?
.
Ethane Lewis Dot Structure would refer to the structure formation of the compound ethane with chemical description. Detailed structure by explaining the facts shown by Lewis structure would be represented in this research. One ethane molecule consists of two carbon and six oxygen atoms. The chemical formula of the molecule is C2H6. The total number of valance electrons in this compound is
C2h6 electron dot structure
Since C has 4 valence electrons , and each H atoms contributes 1 valence electron, the total number of electrons will be. So, the two C atoms are placed in the center of the molecule. Each C atom forms three covalent bonds with three H atoms, with one aditional covalent bond being formed between the two C atoms. Each of these seven single bonds contains 2 electrons, which means that a total of. How can I write the Lewis dot structure for C2H6?
Palm tree sketch easy
The C2H6 Lewis structure is shown below: Steps for drawing the C2H6 Lewis structure Step 1 Calculate the number of valence electrons for C and H Carbon and hydrogen are group 14 and group 1 elements in the periodic table. Write the electron-dot structures for : i ethane, ii ethene, and iii ethyne. Step 4 Stability of structure In Step 3 we can see that the external hydrogen atoms in the C2H6 molecule are forming a bimolecule, so they are stable. Jan 11, Amongst these hybrid orbitals, one hybrid orbital will overlap with the 1s-orbital of the Hydrogen atom that produces the sigma bond between a Hydrogen and Carbon atom. Here, both carbon and hydrogen atom shares their 1 valence electron. How do you draw the lewis structure for ions? See all questions in Drawing Lewis Structures. The molecular geometry of a compound is determined by valance shell electron pair repulsion VSEPR theory. You may like Is sodium a metal or nonmetal? Mar 8,
C2H6, known as ethane, is a saturated open-chain hydrocarbon or we can say that it comes under the alkane family. Hydrocarbon is an organic compound, which contains only carbon and hydrogen.
During bond formation, the orbitals of atoms are hybridized to share electrons with another atom. Impact of this question views around the world. Open in App. Byju's Answer. Does it have to be refrigerated? Step to draw electron dot structure Write down the chemical symbol of the element. What are some common mistakes students make with Lewis structures? The central atom must satisfy the principle of less electronegativity. How do you draw the Lewis structure for ionic compounds? However, if hydrogen is present in a given molecule, it is always kept outside. C2H6 Hybridization During bond formation, the orbitals of atoms are hybridized to share electrons with another atom. How can I write the Lewis dot structure for C2H6?

0 thoughts on “C2h6 electron dot structure”