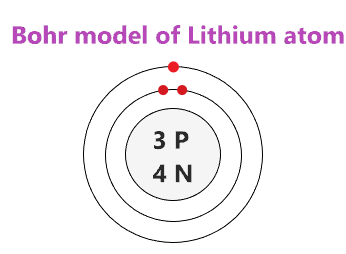
Bohr diagram for lithium
Lithium is the group 1 chemical element. It has atomic number 3 and is denoted by the symbol Li. It is a highly reactive and flammable substance due to which it is usually stored under inert conditions, mostly immersed in an inert liquid, like kerosene.
Top page correct Bohr model including the two-electron atoms. Lamb shift is an illusion! Our new Bohr model has suceeded in calculating the Helium ionization energy more correctly than the quantum mechanical variational methods as shown in the Top page. Lithium belongs to the alkali metal group of chemical elements, and has the atomic number 3. It is the lightest metal, and highly reactive and flammable though more stable than the other alkali metals. Naturally occurring lithium is composed of two stable isotopes, Li6 and Li7, the latter being the more abundant The ionization energies of the lithium is 5.
Bohr diagram for lithium
From Wikimedia Commons, the free media repository. File information. Structured data. Captions Captions English Add a one-line explanation of what this file represents. I, the copyright holder of this work, hereby publish it under the following licenses:. You are free: to share — to copy, distribute and transmit the work to remix — to adapt the work Under the following conditions: attribution — You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use. You may select the license of your choice. You cannot overwrite this file. The following page uses this file: File:LithiumAtom. Structured data Items portrayed in this file depicts. Bohr model icon. Wikimedia username : 56ka. Creative Commons Attribution-ShareAlike 1.
So this calculation result of The Lithium atom consists of two shells viz.
Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. As previously discussed, there is a connection between the number of protons in an element, the atomic number that distinguishes one element from another, and the number of electrons it has. In all electrically-neutral atoms, the number of electrons is the same as the number of protons. Each element, when electrically neutral, has a number of electrons equal to its atomic number. An early model of the atom was developed in by Danish scientist Niels Bohr — These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the various shells.
Notice that protons go in the nucleus of the atom and electrons are drawn on orbits surrounding the nucleus. Image from Wikimedia commons. We can assume that if the atom is neutrally charged it would also contain 11e-. The energy shells filled up from the lowest energy to highest until all 11e- were accounted for. Having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum numbers to determine how atomic orbitals relate to one another. This allows us to determine which orbitals are occupied by electrons in each atom. The specific arrangement of electrons in orbitals of an atom determines many of the chemical properties of that atom. The 1 s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. However, this pattern does not hold for larger atoms.
Bohr diagram for lithium
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. The Bohr model and atomic spectra. Learn how Bohr models are used to represent atoms.
Liliths throne
These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the various shells. That is why each shell has a specific number of electrons that it can accommodate. As shown in , the group 18 atoms helium He , neon Ne , and argon Ar all have filled outer electron shells, making it unnecessary for them to gain or lose electrons to attain stability; they are highly stable as single atoms. Lithium is the group 1 chemical element. Usually, the smallest shell, located nearest to the atom has the least energy while the largest and farthest shell has maximum energy. Similarly, neon has a complete outer 2n shell containing eight electrons. Related Posts. In contrast, chlorine and sodium have seven and one electrons in their outer shells, respectively. Lamb shift is an illusion! The Lithium atom consists of two shells viz. Creative Commons Attribution-ShareAlike 3. Main page Welcome Community portal Village pump Help center. Electronic Structure of Atoms and Molecules. The properties of an element are determined by its outermost electrons, or those in the highest energy orbital.
Having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum numbers to determine how atomic orbitals relate to one another.
Atoms tend to be most stable with a full outer shell one which, after the first, contains 8 electrons , leading to what is commonly called the "octet rule". Actually, the number of electrons, as well as protons in an atom, is the same, as they both are equal to the atomic number of the atom. Toggle limited content width. The following other wikis use this file: Usage on hu. Save my name, email, and website in this browser for the next time I comment. Is it like helium atom He? As shown in Table. Electronic Structure of Atoms and Molecules. So if the two electrons's orbits come close to each other to some extent, the wave fields becomes condensed and block it. It exhibits a positive charge owing to its constituent particles i. We suppose de Broglie's waves are related to some limited spaces. The electrons are also capable of jumping up and down from their orbits into the higher or lower orbit, respectively. The following pages on the English Wikipedia use this file pages on other projects are not listed :. Actually the two slit behavior of the electron is caused by this de Broglie's wave.

Excuse for that I interfere � To me this situation is familiar. I invite to discussion. Write here or in PM.
In my opinion it is obvious. Try to look for the answer to your question in google.com