Bn molecular orbital diagram
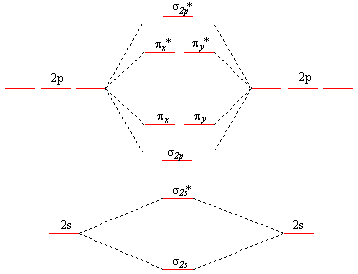
This is the general MO diagram you need to fill with the valence electrons of BN. Boron has 3 valence electronsand nitrogen has 5 valence electrons, bn molecular orbital diagram, this makes 8 electrons. You have to start filling the orbitals from those with lowest energy to those with higher energy.
None of the approaches we have described so far can adequately explain why some compounds are colored and others are not, why some substances with unpaired electrons are stable, and why others are effective semiconductors. These approaches also cannot describe the nature of resonance. Such limitations led to the development of a new approach to bonding in which electrons are not viewed as being localized between the nuclei of bonded atoms but are instead delocalized throughout the entire molecule. Just as with the valence bond theory, the approach we are about to discuss is based on a quantum mechanical model. In Chapter 2 , we described the electrons in isolated atoms as having certain spatial distributions, called orbitals , each with a particular orbital energy.
Bn molecular orbital diagram
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs. Extensive Properties. Scientific Notation. Metric Prefixes. Significant Figures. Significant Figures: Precision in Measurements. Significant Figures: In Calculations. Conversion Factors.
Periodic Trend: Ionization Energy.
.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs. Extensive Properties. Scientific Notation.
Bn molecular orbital diagram
Valence bond theory is able to explain many aspects of bonding, but not all. To complement this theory, we use another called the molecular orbital MO theory. Molecular orbital theory is a more sophisticated model for understanding the nature of chemical bonding. MO theory takes the idea of atomic orbitals overlapping to a new level, where new molecular orbitals are generated using a mathematical process called linear combination of atomic orbitals LCAO. The major difference between atomic and molecular orbitals is that atomic orbitals represent electron density in space associated with a particular atom. Molecular orbitals are associated with the entire molecule, meaning the electron density is delocalized spread out over more than one atom.
Le castle river hotel & apartment
Is this ion likely to be a stable species? Molecular Orbitals Involving Only ns Atomic Orbitals We begin our discussion of molecular orbitals with the simplest molecule, H 2 , formed from two isolated hydrogen atoms, each with a 1 s 1 electron configuration. Gases 3h 54m. The Electron Configuration. In the solid state, however, all the alkali metals and the alkaline earth metals exist as extended lattices held together by metallic bonding. Chemical Kinetics 2h 42m. Scientific Notation. Lewis Acids and Bases. Constant-Pressure Calorimetry. The Ideal Gas Law. Bronsted-Lowry Acids and Bases. Combustion Apparatus.
None of the approaches we have described so far can adequately explain why some compounds are colored and others are not, why some substances with unpaired electrons are stable, and why others are effective semiconductors.
Boron has 3 valence electrons , and nitrogen has 5 valence electrons, this makes 8 electrons. As the overlap between two atomic orbitals increases, the difference in energy between the resulting bonding and antibonding molecular orbitals increases. To determine what type of bonding the molecular orbital approach predicts F 2 to have, we must calculate the bond order. Intro to Acid-Base Titration Curves. None of the approaches we have described so far can adequately explain why some compounds are colored and others are not, why some substances with unpaired electrons are stable, and why others are effective semiconductors. Hence the electron density of bonding electrons is likely to be closer to the more electronegative atom. How do Lewis electron structures represent nonbonding electrons? In this combination, shown in part b in Figure 5. Such limitations led to the development of a new approach to bonding in which electrons are not viewed as being localized between the nuclei of bonded atoms but are instead delocalized throughout the entire molecule. Average Bond Order. Stoichiometric Rate Calculations.

It is remarkable, a useful idea