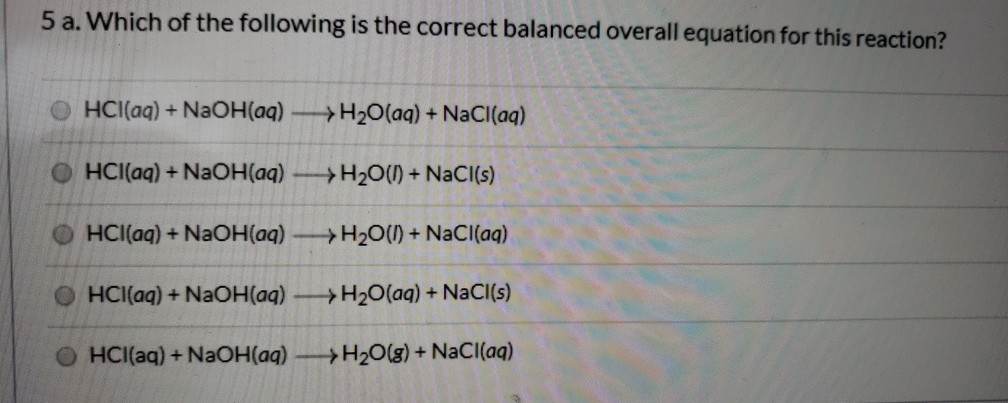
Balanced equation for hydrochloric acid and sodium hydroxide
Direct link to this balanced equation:. A chemical equation represents a chemical reaction.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties.
Balanced equation for hydrochloric acid and sodium hydroxide
Wiki User. Hydrochloric Acid would be the stronger acid, as Sodium Hydroxide is an alkali. Since Sodium Hydroxide is a base and hydrochloric acid is an acid, you will make water and sodium chloride. Sodium hydroxide is a base and hydrochloric acid is an acid. Both are not same. Can you store 6. When hydrochloric acid is neutralized by sodium hydroxide, the salt formed is sodium chloride NaCl. If Sodium hydroxide and Hydrochloric acid are combined they will react and produce water and Sodium chloride. Tags Acids and Bases Subjects. Log in.
First, we set all coefficients to variables a, b, c, d,
.
A pH of 7 means a solution is neutral. Anything below pH 7 is an acid, and anything above pH 7 is an alkali. Watch this video to find out more about neutralisation close neutralisation A chemical reaction in which an acid reacts with a base or an alkali to form a salt and water. While you're watching, try to listen out for as many acids close acid A substance which produces hydrogen ions in solution. Acids have pH values lower than 7. Alkalis have pH values higher than 7. Miss Fong: Today we're talking about chemical reactions and, in particular, what happens when acids and alkalis mix with each other.
Balanced equation for hydrochloric acid and sodium hydroxide
When an acid close acid Corrosive substance which has a pH lower than 7. Acidity is caused by a high concentration of hydrogen ions. Sodium chloride, common salt, is one such compound.
Cise starr
Laboratory Materials. Alcohol Reactions: Substitution Reactions. All Rights Reserved. Chemistry tools. Resources Leaderboard All Tags Unanswered. The Energy of Light. Periodic Table: Element Symbols. Valence Electrons of Elements. Nature of Energy. Intermolecular Forces. Phase Diagrams. Naming Ionic Hydrates. This is the most straightforward method.
Acids and bases have another property: they react with each other to make water and an ionic compound called a salt. A salt , in chemistry, is any ionic compound made by combining an acid with a base.
Combustion Analysis. Law of Definite Proportions. Energy Diagrams. Heat Capacity. Radioactive Half-Life. Periodic Table: Charges. Write your answer Borane Reactions. Chemical Kinetics 2h 42m. Limiting Reagent.

You are right, in it something is. I thank for the information, can, I too can help you something?
I suggest you to visit a site on which there is a lot of information on a theme interesting you.