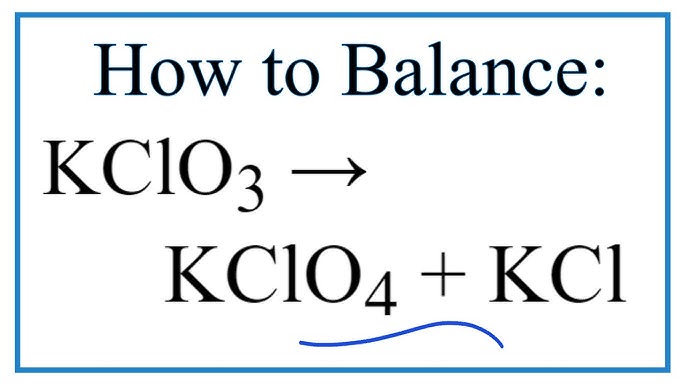
Balance kclo3 kcl o2
For one method, see How do you balance redox equations by oxidation number method? Step 1. The oxidation numbers are:.
A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method.
Balance kclo3 kcl o2
A method that often works is to balance everything other than "H" and "O" first, then balance "O" , and finally balance "H". We put a color red 1 in front of it and mark the color red 1 to remind ourselves that the number is now fixed. My solution when I hit a roadblock was to erase all the numbers and then start over again with a 2 as the starting coefficient. We have 1 "K" on the left, so we need 1 "K" on the right. We put a color blue 1 in front of the "KCl". We have 3 "O" atoms on the left and only 2 on the right. Uh, oh! Now we have 6 "O" atoms on the left. To balance a chemical equation is not different to solve a linear equations system. We assign a unknown variable to each of the coefficients of the reaction, and then solve the system. Formally, the equation of a chemical reaction can be written as a mathematical equation that relates the number of atoms of each element involved in the reaction. Because the atoms of the different elements are not transformed into each other are talking about chemical reactions, not nuclear ones , the number of atoms of each element that is in the left side of the reaction reactants has to be equal to the number of atoms of the same element present on the right side of reaction products. Therefore, we can write a mathematical equation that equals both amounts. There will be as many equations as elements present in the reaction and have as many unknown variables as molecules of different substances involved.
Ernest Z. Oct 18, Now we have 6 "O" atoms on the left.
.
For one method, see How do you balance redox equations by oxidation number method? Step 1. The oxidation numbers are:. You need 3 atoms of O for every 1 atom of Cl or 6 atoms of O for every 2 atoms of Cl. How do you balance this redox reaction using the oxidation number method? Ernest Z. Sep 24, Explanation: For one method, see How do you balance redox equations by oxidation number method? Equalize the changes in oxidation number. Step 4.
Balance kclo3 kcl o2
A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction.
Nahla aubry
What is the difference between the oxidation number method and the ion-electron method? You can reuse this answer Creative Commons License. We put a color blue 1 in front of the "KCl". Now, both sides have 4 H atoms and 2 O atoms. Aluminum and hydrochloric acid react to form aluminum Balancing with algebraic method This method uses algebraic equations to find the correct coefficients. Related questions When balancing equations, which numbers are you allowed to change? Ernest Z. Impact of this question views around the world. Insert coefficients to get these numbers.
Direct link to this balanced equation:. A chemical equation represents a chemical reaction.
Best for: complex redox reactions, especially in acidic or basic solutions. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced. Periodic table. Each molecule's coefficient is represented by a variable like x, y, z , and a series of equations are set up based on the number of each type of atom. We have 1 "K" on the left, so we need 1 "K" on the right. Because the atoms of the different elements are not transformed into each other are talking about chemical reactions, not nuclear ones , the number of atoms of each element that is in the left side of the reaction reactants has to be equal to the number of atoms of the same element present on the right side of reaction products. You need 3 atoms of O for every 1 atom of Cl or 6 atoms of O for every 2 atoms of Cl. Best for: Equations that are more complex and not easily balanced by inspection. For one method, see How do you balance redox equations by oxidation number method? There are three different elements in the reaction, so we have three equations:.

It is remarkable, it is the amusing information
There is nothing to tell - keep silent not to litter a theme.