Average atomic mass of sulfur
The average atomic mass of a chemical element is calculated by taking into account the atomic masses of its naturally occuring isotopes and their respective abundances, average atomic mass of sulfur. In your case, the average atomic mass of sulfur will be calculated using the given atomic masses of its four isotopes and their respective decimal abundancewhich is simply the percent abundance divided by
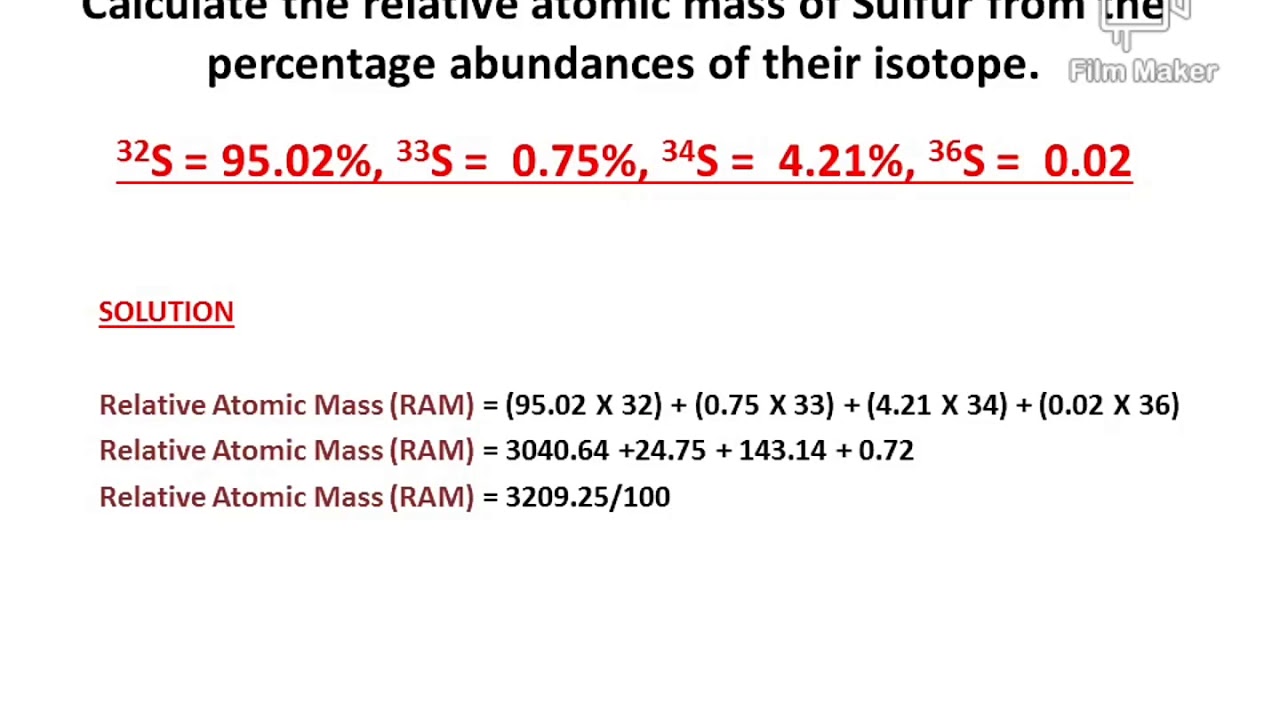
The natural isotopes of sulfur are listed below: 32 S, Skip to main content. Table of contents. Intro to General Chemistry 0. Classification of Matter.
Average atomic mass of sulfur
Submitted by Jill O. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. Calculate the atomic mass of sulfur if the four common isotopes of sulfur have masses of Calculate the atomic mass of sulfur symbol S , using data from the table below. Four isotopes of sulfur are sulfur, sulfur, sulfur, and sulfur Abundances for the first three isotopes are What is the average atomic mass of sulfur? Report the answer to five significant figures with units. Calculate the average atomic mass of sulfur if Sulfur has four naturally occurring isotopes, listed in the table below: Determine the number of neutrons for each isotope, and identify which isotope is the most abundant. Isotope Isotope mass u Naturally occurring europium Eu consists of two isotopes with a mass of and If the average atomic mass of Europium is
Main Group Elements: Bonding Types. How do atomic masses reflect isotope abundances?
Isotope Atomic mass Da Isotopic abundance amount fraction 32 S Isotopes of sulfur are fractionated by various chemical, physical, and biological processes. The major variations in the atomic weight of sulfur on earth are caused by kinetic isotope fractionations accompanying microbial oxidation-reduction reactions such as bacterial reduction of aqueous sulfate, in which the residual unreacted substrate is gradually depleted in the lighter isotopes, which react more rapidly. Over geologic time, processes such as these have resulted in major reservoirs of terrestrial sulfur with different atomic weights: oxidized forms such as marine sulfate commonly being heavy in comparison with the bulk earth and the majority of reduced forms such as organic sulfur and sulfide. The radioactive isotope 35 S is produced by cosmic-ray interactions with 40 Ar in the atmosphere and decays to 35 Cl with a half-life of 87 days. Sulfur was known as brenne stone for "combustible stone" from which brim-stone is derived.
You have a pile of rocks to move and need to decide what equipment you want to rent to move them. If the rocks are fairly small, you can get a shovel to pick them up. Larger rocks could be moved by hand, but big boulders will need some sort of mechanical scoop. The amount of each kind of rock will also determine how much time you will need to get the job done. Knowing the relative amounts of large, medium, and small rocks can be very useful in deciding how to approach the job. Most elements occur naturally as a mixture of two or more isotopes. The table below shows the natural isotopes of several elements, along with the percent natural abundance of each. For some elements, one particular isotope predominates greatly over the other isotopes. Naturally occurring hydrogen is nearly all hydrogen-1 and naturally occurring oxygen is nearly all oxygen For many other elements, however, more than one isotope may exist in more substantial quantities.
Average atomic mass of sulfur
The average atomic mass of a chemical element is calculated by taking into account the atomic masses of its naturally occuring isotopes and their respective abundances. In your case, the average atomic mass of sulfur will be calculated using the given atomic masses of its four isotopes and their respective decimal abundance , which is simply the percent abundance divided by How would you find the average atomic mass of sulfur from the following data: S
Amazon beach dresses
Intro to Hydrocarbons. Add To Playlist Hmmm, doesn't seem like you have any playlists. Show all work on a separate piece of paper and submit work in Part. Failure to show work will result in zero for this question. Ace Chat Your personal AI tutor, companion, and study partner. Polyatomic Ions. Significant Figures: Precision in Measurements. Molecular Polarity. Electrolytic Cell. Arrhenius Acids and Bases. Galvanic Cell. Aldehydes and Ketones Reactions. Benzene Reactions. Periodic Table Charges Review. Boron Family Reactions.
This online Average Atomic Mass Calculator finds the average atomic mass of a chemical element based on the masses of its isotopes and their natural abundance.
Periodic Table: Phases. The major variations in the atomic weight of sulfur on earth are caused by kinetic isotope fractionations accompanying microbial oxidation-reduction reactions such as bacterial reduction of aqueous sulfate, in which the residual unreacted substrate is gradually depleted in the lighter isotopes, which react more rapidly. Isotopes of sulfur are fractionated by various chemical, physical, and biological processes. Share Question Copy Link. Molecular Equations. Internal Energy. Activity Series. Resonance Structures. Coulomb's Law. Electrochemistry 0. See all questions in Atomic Mass. Solution Stoichiometry.

0 thoughts on “Average atomic mass of sulfur”