Aluminium sulfate ionic formula
Al 2 SO 4 3 is a chemical compound with the chemical name Aluminium sulphate. Aluminium sulphate is also called Filter Alum or Dialuminum trisulphate.
Aluminium sulfate is a double sulfate salt of aluminium usually available in hydrated form. This is an Inorganic salt that is made from the neutralization reaction of Aluminium Hydroxide and sulfuric acid. It is an edible substance used for drinking water purification, as a pickling agent, and as one of the components in baking powder. Aluminium sulfate is also used as a chemical that enhances the immune response. It reduces the growth of bacteria on the skin. Aluminium sulfate is also used as a preservative. However, Using it in excess may lead to some skin irritations also.
Aluminium sulfate ionic formula
.
However, Using it in excess may lead to some skin irritations also. So, using Aluminium sulfate, which is an antiseptic, astringent and anti-bacterial, helps to avoid some skin-related issues.
.
Explore the properties, applications, potential hazards, and environmental implications of aluminum sulfate in this comprehensive guide. Aluminum sulfate, often referred to as alum, is a chemical compound with the formula Al 2 SO 4 3. This versatile compound has a wide range of applications in various industries due to its distinctive physical and chemical properties. Aluminum sulfate is commonly prepared in an industrial setting by reacting aluminum hydroxide, Al OH 3 , with sulfuric acid, H 2 SO 4. This reaction results in the production of aluminum sulfate and water. Aluminum sulfate forms colorless crystals that are hygroscopic, meaning they readily absorb moisture from the air. It is soluble in water and its solutions are acidic due to the presence of the sulfate ion. The compound has a sweet, mildly astringent taste, although it is not typically used in food or ingested due to its toxicity in larger quantities. Water Treatment: Aluminum sulfate is extensively used in water treatment plants to promote coagulation, facilitating the removal of small particles suspended in the water. It reacts with bicarbonate alkalinity present in water to form an insoluble aluminum hydroxide precipitate, which can be easily filtered out.
Aluminium sulfate ionic formula
We have already encountered some chemical formulas for simple ionic compounds. A chemical formula is a concise list of the elements in a compound and the ratios of these elements. To better understand what a chemical formula means, we must consider how an ionic compound is constructed from its ions. However, we can use the ratio of sodium ions to chloride ions, expressed in the lowest possible whole numbers, as a way of describing the compound. A macroscopic sample is composed of myriads of NaCl pairs; each individual pair called a formula unit or empirical formula. The formula unit or empirical formula represents the minimum proportion between cations and anions in the crystal lattice. Each ion is surrounded by ions of opposite charge.
Graphic designer salary
It makes the cement mix correctly without any leaks. We hope this article has given the readers insight into the topic of aluminium sulfate chemistry. Aluminium sulfate is usually available in hydrated form and is referred to as Alum. It is soluble in water and is primarily used in purification of drinking water and wastewater treatment plants as a coagulating agent promoting particle collision by neutralizing charge as well as in paper processing. Aluminium sulfate is used as a preservative, as a coagulating agent, as a component in the manufacture of paper and baking soda, for the printing and dyeing of fabrics, etc. The solution of aluminium sulphate is corrosive to aluminium. Chloroform Formula. On decomposing, it emits toxic fumes of sulphur oxides. It is mildly dangerous if aluminium sulphate is swallowed in any way because when the salt is swallowed it can form extremely corrosive sulphuric acid. Explore SuperCoaching Now.
Aluminium sulfate is a salt with the formula Al 2 SO 4 3. It is soluble in water and is mainly used as a coagulating agent promoting particle collision by neutralizing charge in the purification of drinking water [3] [4] and wastewater treatment plants , and also in paper manufacturing. The anhydrous form occurs naturally as a rare mineral millosevichite , found for example in volcanic environments and on burning coal-mining waste dumps.
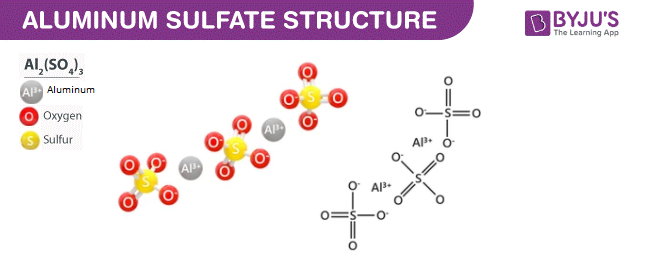
What Is Precipitation. In Sewage treatment Aluminium sulfate is an inorganic salt that is widely used in wastewater treatment as a Coagulant. Is Aluminium sulfate soluble in water? Each Aluminium ion is bonded to the two oxides, each of the two sulfate ions like a bridge. What is the smell of Aluminium sulphate? Al 2 SO 4 3 is a chemical compound with the chemical name Aluminium sulphate. Both the forms are non-toxic and non-combustible. Why is aluminium sulphate soluble in water? Aluminium sulfate is used as a preservative, as a coagulating agent, as a component in the manufacture of paper and baking soda, for the printing and dyeing of fabrics, etc. Download as PDF.

Also that we would do without your brilliant phrase