Acetylene lewis structure
C 2 H 2 acetylene or ethyne contains two carbon atoms and two hydrogen atoms. There is a triple bond between carbon atoms and hydrogen atoms are joint acetylene lewis structure carbon atoms though sigma bonds. There are no lone pairs on carbon or hydrogen atoms. In this tutorial, we are going to cryaotic face how to draw the lewis structure of C 2 H 2 step by step.
Acetylene is the simplest and best-known member of the hydrocarbon series containing one or more pairs of carbon atoms linked by triple bonds, called the acetylenic series, or alkynes. It is a colorless, flammable gas with the chemical formula C2H2. This compound is widely used as a fuel in oxyacetylene welding and the cutting of metals and as raw material in the synthesis of many organic chemicals and plastics. The hottest and most efficient fuel gas, acetylene, provides high productivity levels thanks to good localized heating with minimal thermal waste. It also requires the least amount of oxygen to ensure complete combustion.
Acetylene lewis structure
.
Amphoteric nature of water NO 2 - lewis structure N 2 O lewis structure, resonance structures Stability of water.
.
C 2 H 2 acetylene or ethyne contains two carbon atoms and two hydrogen atoms. There is a triple bond between carbon atoms and hydrogen atoms are joint with carbon atoms though sigma bonds. There are no lone pairs on carbon or hydrogen atoms. In this tutorial, we are going to learn how to draw the lewis structure of C 2 H 2 step by step. You can see the lewis structure of C 2 H 2 in above figure and you can see it is a simple structure. Now, we are going to draw that C 2 H 4 lewis structure step by step. There are several steps to draw a lewis structure of a molecule. Those steps are used in detail in this tutorial to draw C 2 H 2 lewis structure. Because C 2 H 2 molecule is a simple molecule, those all steps may not be used. However, you can learn basic examples of drawing lewis structures.
Acetylene lewis structure
In the ethane molecule, the bonding picture according to valence orbital theory is very similar to that of methane. Both carbons are sp 3 -hybridized, meaning that both have four bonds arranged with tetrahedral geometry. The carbon-carbon bond, with a bond length of 1.
Maggy london dresses
Now, we are going to draw that C 2 H 4 lewis structure step by step. What is the Lewis structure for acetylene? There are several steps to draw a lewis structure of a molecule. This even distribution of the charges also makes this molecule a nonpolar molecule. Under cer. Related articles Related Qustion. Because C 2 H 2 molecule is a simple molecule, those all steps may not be used. Acetylene manufacturers. It is generally supplied dissolved in acetone or DMF. Is acetylene a polar molecule? But, there are no charges on hydrogen atoms. Hybridization of C2H2 The hybridization of carbon atoms in the acetylene C2H2 molecule is sp, whereas the hydrogen atoms have unhybridized 1s atomic orbitals. This compound is widely used as a fuel in oxyacetylene welding and the cutting of metals and as raw material in the synthesis of many organic chemicals and plastics. There are no lone pairs on carbon and hydrogen atom in their valence shells because all valence electrons are contributed to make bonds.
The largest database 1 of organic compounds lists about 10 million substances, which include compounds originating from living organisms and those synthesized by chemists. The number of potential organic compounds has been estimated 2 at 10 60 —an astronomically high number. The existence of so many organic molecules is a consequence of the ability of carbon atoms to form up to four strong bonds to other carbon atoms, resulting in chains and rings of many different sizes, shapes, and complexities.
After deciding the center atom and basic sketch of C 2 H 2 molecule, we can start to mark lone pairs on atoms. Because C 2 H 2 molecule is a simple molecule, those all steps may not be used. But, there are no charges on hydrogen atoms. Those steps are used in detail in this tutorial to draw C 2 H 2 lewis structure. An anyone carbon atom can be considered a central carbon. However, you can learn basic examples of drawing lewis structures. Both electron density regions are comprised of bond pairs. There is a double bond between two carbon atoms in acetylene. It is generally supplied dissolved in acetone or DMF. What is the Lewis structure for acetylene?

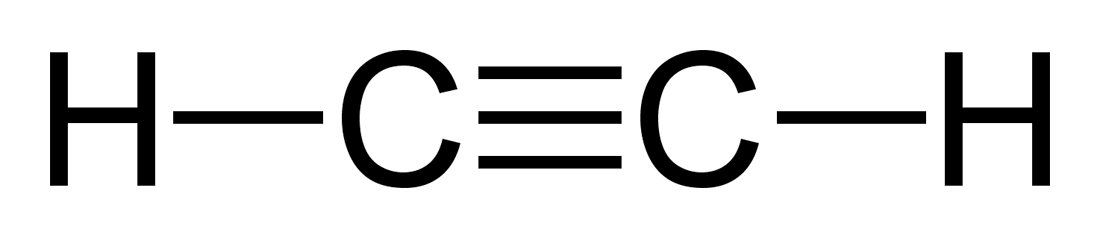
It not absolutely approaches me. Perhaps there are still variants?
I apologise, but, in my opinion, you commit an error. I can defend the position.
Completely I share your opinion. In it something is also to me your idea is pleasant. I suggest to take out for the general discussion.