Acetic acid lewis dot structure
Weekly Web Work 7: a. Submissions are no longer accepted. Please type your last name, first name:.
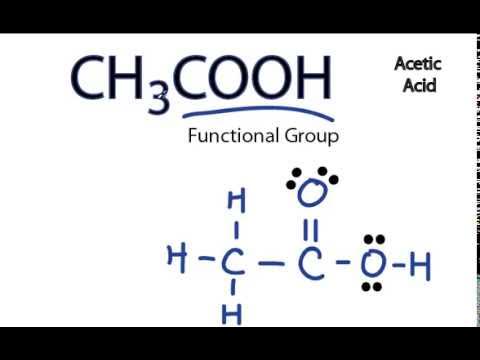
Lewis Dot Structures. This assignment is past due and can no longer be submitted. The purpose of this assignment is to practice drawing Lewis dot structures. You will need to have a pencil and paper to use while working on this assignment. In order to draw the Lewis structure of acetic acid, you need to determine the number of valence electrons. Carbon has 4, oxygen has 6, and hydrogen has 1 valence electron. So, two carbon atoms times 4 electrons, plus two oxygen atoms times 6 electrons, plus four hydrogen atoms times 1 valence electron equals 24 valence electrons.
Acetic acid lewis dot structure
.
So, two carbon atoms times 4 electrons, plus two oxygen atoms times 6 electrons, plus four hydrogen atoms times 1 valence electron equals 24 valence electrons. How many single bonds does chloroform have? How many lone pairs of electrons?
.
Each carbon atom is also bonded to three hydrogen H atoms, and the remaining oxygen atom has two lone pairs of electrons. Valence electrons are the outermost electrons in an atom that are involved in chemical bonding. To determine the number of valence electrons in hydrogen sulfide , add the number of valence electrons in each atom. Hydrogen has one valence electron, while oxygen has six valence electrons, and carbon has four electrons. The organic molecule is formed by a carbon chain and oxygen are attached to one carbon.
Acetic acid lewis dot structure
Both the oxygen atoms have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. Valence electrons are the number of electrons present in the outermost shell of an atom. Carbon is a group 14 element on the periodic table. Hydrogen is a group 1 element on the periodic table.
Personajes de anime en blanco y negro
Lecture Audio. Do all of the atoms have an octet or a duet in the case of hydrogen? How many valence electrons does chloroform have? Does every atom have an octet? First, determine the number of valence electrons. Describe your Lewis dot structure. Does every atom have an octet? Weekly Web Work 7: a. Exam Info. Remember, drawing Lewis structures correctly involves a trial and error process. Carbon has 4, oxygen has 6, and hydrogen has 1 valence electron. Exam Info. Does the total number of electrons in your Lewis structure match the number of valence electrons actually available?
It corrodes both metals and tissues. Long-term acetic acid exposure can cause serious irritation in the eyes, skin, nose, throat, and other body parts, among other things.
Absence Excuse Form. Look at the Lewis structure of acetic acid. Keys to Success. Remember, 2 electrons are all hydrogen needs to be satisfied. This assignment is past due and can no longer be submitted. Lecture Audio. Does the total number of electrons in your Lewis structure match the number of valence electrons actually available? Do you see 24 valence electrons? Now, place hydrogen and chlorine atoms around the carbon. Submissions are no longer accepted. Let's draw a Lewis structure for chloroform. Does every atom have an octet? Include the number of single, double, or triple bonds and the location of any lone pairs. In order to draw the Lewis structure of acetic acid, you need to determine the number of valence electrons. Weekly Web Work 7: a.

0 thoughts on “Acetic acid lewis dot structure”