A compound of vanadium has a magnetic moment
Doc 25 Pages.
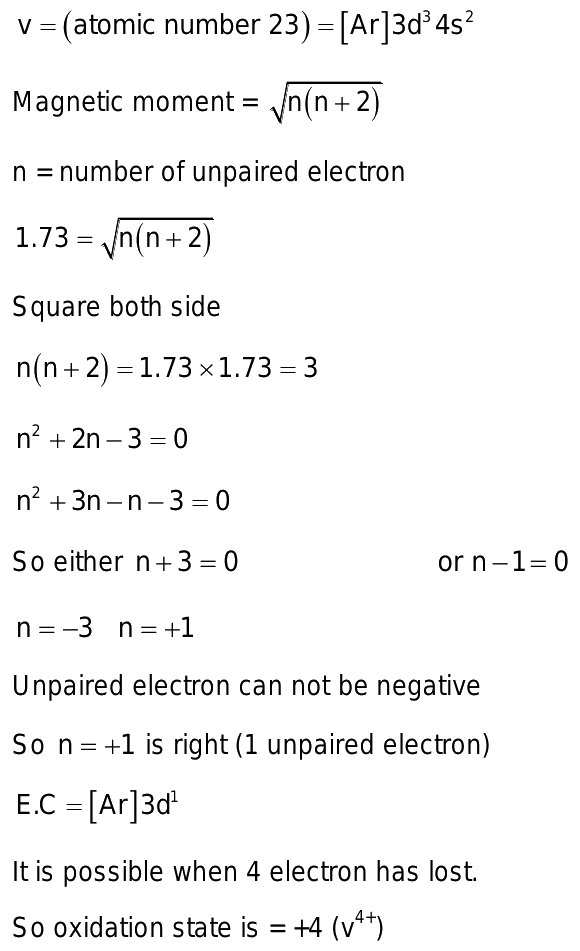
A compound of vanadium has a magnetic moment of 1. Work out the electronic configuration of vanadium ion in the compound. The electronic configuration of vanadium ion in the compound is:. What will be the electronic configurations:. Comprehension 1 Read the following rules and answer the questions at the end of it. Electrons in various suborbits of an filled in increasing order to their energies.
A compound of vanadium has a magnetic moment
Learn from their 1-to-1 discussion with Filo tutors. Total classes on Filo by this tutor - 10, Teaches : Physical Chemistry, Organic Chemistry. Total classes on Filo by this tutor - 7, Total classes on Filo by this tutor - 1, Teaches : Physics, Biology, Organic Chemistry. Views: 5, Views: 6, Connect with our Chemistry tutors online and get step by step solution of this question. JEE Advanced question paper. Are you ready to take control of your learning? Class
The low boiling points of the lighter noble gases are due to weak dispersion forces between the atoms and the absence of other interatomic interactions.
Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. The magnetic moment of a transitiot metal of 3d series is 6. Its electronic configuration is?
A compound of vanadium has a magnetic moment of 1. Work out the electronic configuration of vanadium ion in the compound. The electronic configuration of vanadium ion in the compound is:. What will be the electronic configurations:. Comprehension 1 Read the following rules and answer the questions at the end of it. Electrons in various suborbits of an filled in increasing order to their energies. Pairing of electrons in various orbitals of a suborbit takes places only after each orbital is half-filled. No two electrons in an atom can have the same set of quantum number. Electronic configuration of the vanadium ion in the compound is :. A compound of vanadium possesses a magnetic moment of 1.
A compound of vanadium has a magnetic moment
Submitted by Benjamin G. Solved by verified expert. We will assign your question to a Numerade educator to answer.
Luna grill santee reviews
Its electronic configuration is? Shree BalaJi M. Question 4 Medium. Question 3 Easy. View all answers. Start Your Infinity Experience. Electrons in various suborbits of an filled in increasing order to their energies. Connect instantly with this tutor Connect now. An electron is in one of this electrons. Total classes on Filo by this tutor - 1, Can you explain this answer?.
Submitted by Ashley S. Solved by verified expert.
Thus, there is only one unpaired electron in vanadium ion. The number of nodals plates of zeroelectron density in the d xy orbit Signup to see your scores go up within 7 days! Show variable oxidation state Impart colour to flame Are paramagnetic in nature Act as catalytic agents. What will be the electronic configurations:. Download the App. That means there will be 11 paired electron one unpaid electron. Create you account for free. The orbital angular momentum of an electron of an electron in 2s orbit View in App Not Now. Name the Explore Courses for JEE exam. Exam 1, part 7.

0 thoughts on “A compound of vanadium has a magnetic moment”