2agcl
Silver chloride is an inorganic chemical compound with the chemical formula Ag Cl. 2agcl white 2agcl solid is well known for its low solubility in water and its sensitivity to light. Upon illumination or heating, silver chloride converts to silver and chlorine2agcl, 2agcl, which is signaled by grey to black or purplish coloration in some samples.
Then, lookup atomic weights for each element in periodic table : Ba: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:.
2agcl
.
Unlike photography, 2agcl the photoreduction is irreversible, the glass prevents the electron from being 'trapped'. Upon illumination or heating, silver chloride converts to silver and chlorinewhich is signaled by grey to black or purplish coloration in some samples, 2agcl. Studies in Conservation.
.
Silver chloride is an inorganic chemical compound with the chemical formula Ag Cl. This white crystalline solid is well known for its low solubility in water and its sensitivity to light. Upon illumination or heating, silver chloride converts to silver and chlorine , which is signaled by grey to black or purplish coloration in some samples. AgCl occurs naturally as the mineral chlorargyrite. It is produced by a metathesis reaction for use in photography and in pH meters as electrodes. Silver chloride is unusual in that, unlike most chloride salts, it has very low solubility. It is easily synthesized by metathesis : combining an aqueous solution of silver nitrate which is soluble with a soluble chloride salt, such as sodium chloride which is used industrially as a method of producing AgCl , or cobalt II chloride.
2agcl
Well you gots the stoichiometric equation And silver chloride is as soluble as a BRICK, and precipitates from aqueous solution as a curdy white solid. When you try to collect the silver chloride it is a very curdy, unhandy precipitate. And yet, at one stage, given the photosensitivity of the silver chloride which sometimes you can see , plates of silver chloride were once used as the basis for black and white photography.
Yify subs
Other anions. CfCl 3. Calculate molar mass of each element: multiply the atomic mass of each element by the number of atoms of that element in the compound. Find atomic masses: look up the atomic masses of each element present in the compound. Retrieved 25 February PMC CuCl CuCl 2. In other projects. AgCl quickly darkens on exposure to light by disintegrating into elemental chlorine and metallic silver. PtCl 2 PtCl 4. Silver compounds. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. Gas laws. Greenwood; A. Space group.
Silver chloride is a white crystalline chemical compound with the formula AgCl. Silver chloride in the test tube quickly turns purplish, especially in a sunny laboratory because the silver chloride is split up into silver and chlorine. Silver chloride is prepared when sodium chloride is added to silver nitrate solution a white precipitate of silver chloride occurs.
Examples of molecular weight computations: C[14]O[16]2 , S[34]O[16]2. Silver chloride is unusual in that, unlike most chloride salts, it has very low solubility. NdCl 2 NdCl 3. Interactive image. The molar mass of carbon dioxide is GaCl GaCl 3. Microscopy Research and Technique. Silver chloride is a constituent of the silver chloride electrode which is a common reference electrode in electrochemistry. ICl ICl 3. ISBN WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. In general, silver chloride is antimicrobial against various bacteria , such as E. CAS Number.

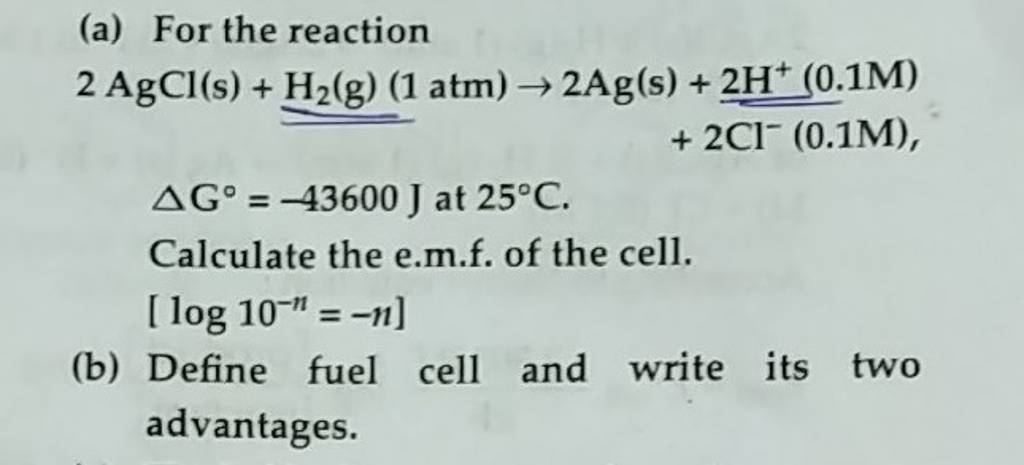
I congratulate, a brilliant idea
I suggest you to come on a site where there is a lot of information on a theme interesting you.